Abstract
Struvite calculi, composed of magnesium ammonium phosphate, have existed for thousands of years in human medicine and are a leading cause of calculi in companion animals. Struvite stones have also been called urease, infection-induced, phosphatic, and triple phosphate stones. They are the most common uroliths in dogs, in which most cases of struvite urolithiasis are associated with infection. Management of struvite urolithiasis requires a multimodal approach that addresses the presence of the urolith(s) and associated infection while identifying risk factors that predispose to the development of infection.
Key Points
- Struvite calculi are usually associated with bacterial urease production in dogs.
- Effective medical dissolution therapy exists for struvite calculi.
- Antibiotic administration during dissolution therapy is usually necessary for success.
- Preventive efforts for struvite calculi should address underlying causes for infection.
- Routine monitoring for urinary tract infections is crucial to prevention of struvite calculi.
Pathophysiology
Struvite stone constituents exist within normal urine, but stone formation depends on diet, local urinary microenvironment, metabolic factors, and concurrent therapy. The precipitation-crystallization theory of stone formation likely plays a role in struvite urolithiasis.1 In this theory, supersaturation of urine with ions results in precipitation and formation of particles. Particles grow into small crystals and aggregate into larger crystals. Ultimately, calculi form if the microenvironment favors this process.
Urine pH can alter struvite crystal solubility, with acidic (pH <6.3) urine resulting in dissolution of struvite crystals, whereas neutral and alkaline (pH >7.0) urine favor struvite crystal formation. Dietary factors may alter crystal solubility and urinary constituent concentrations. These factors may include a lower anion-cation balance of the diet and/or reduced concentrations of magnesium, phosphorus, or sulfur.2 Protein catabolism can alter urine pH and crystal solubility, increase urea production, and decrease phosphorus and magnesium excretion, which can lead to stone formation. Local urinary factors, including damage to uroendothelial glycosaminoglycans, may promote struvite crystal growth.3 Additionally, sex may affect the likelihood of struvite urolithiasis, with females having higher predisposition for infections.4 Rare risk factors can promote struvite urolithiasis; for example, a foreign body can act as a nidus for precipitation and aggregation of crystals.5
Despite the factors mentioned above, struvite urolithiasis formation is unlikely without concurrent production of the enzyme urease, which is produced by certain bacterial species ().
Urease acts to convert urea into ammonia in the presence of water. Generated ammonia (NH3) is free to buffer hydrogen ions (H+) generated from production of carbonate and conversion of phosphorus to phosphate ions, resulting in the formation of ammonium (NH4+). As the hydrogen ions are buffered, the urinary pH rises, contributing to struvite crystallogenesis through reduction in struvite crystal solubility.6 Ammonium also contributes to crystallogenesis because it is a component of struvite uroliths (MgNH4 PO4 6H2O). Ammonium also can induce local glycosaminoglycan damage that may facilitate crystal and bacterial uroendothelial attachment.7
Common findings on urinalysis include hematuria, pyuria, bacteruria, and proteinuria. The urine pH is frequently neutral to alkaline, and struvite crystalluria may be present. Struvite crystalluria does not definitively result in struvite calculi.8 Additionally, iatrogenic struvite crystalluria is possible if urine samples are refrigerated or not run within 60 minutes.9 Given the association with infection, urine should optimally be collected via cystocentesis following appropriate clipping and disinfection of the skin and placed into appropriate transport media or a sterile container for rapid plating.
Struvite urolithiasis in dogs without concurrenturopathogenic urease production or sterile struvite calculi has been reported.1 Its pathophysiology has not been fully elucidated. Distal renal tubular acidosis has been associated with sterile urolithiasis in people and in a study of related cocker spaniels.10
Microbiology
The most common urease-producing bacteria associated with struvite urolithiasis in dogs are Staphylococcus pseudintermedius and Proteus spp.11 Bacteria that occasionally produce urease include Pseudomonas spp, Klebsiella spp, and Escherichia coli ().1
Signalment
Females have been overrepresented in multiple studies of struvite urolithiasis in both human and veterinary medicine.3,11,12 This may be explained by the increased prevalence of urinary tract infections in female patients.
The mean age of veterinary patients is 3 to 7 years, with a wide range from 1 month to 19 years.13,14 Evidence points toward younger animals (<5 years) having an increased relative risk.15 The cause for this trend is unknown.
Breed predispositions have been described (). Ling et al15,16 suggest that not only breed but also age and sex contribute to struvite urolithiasis predisposition. They found that male Labrador retrievers over 10 years of age, male pugs regardless of age, and cocker spaniels between 6 and 10 years of age, regardless of sex, were at increased risk.15,17
Anatomic Location
In dogs, struvite urolithiasis is most common (95%) in the lower urinary tract, with approximately 5% of stones reported within the upper urinary tract.3 Roughly 29% to 33% of renal calculi and 42% of ureteral calculi are composed of struvite exclusively.16,18–20 In comparison, humans frequently have struvite nephrolithiasis or ureterolithiasis, with a smaller percentage having cystic calculi.1
Incidence
Geographic region has a notable effect on incidence, but struvite uroliths remain the most common canine uroliths in multiple studies. Of all urolith submissions, struvite accounts for 50% of submitted uroliths in the United States, 52% of submissions in Ireland, 44% of submissions in Canada, and 39% of submissions in the Czech Republic.3,12,13,21
The incidence of struvite calculi has declined gradually over the past 30 years, with a more abrupt decline between 1981 and 1998 before reaching a plateau.17 The decline in incidence of struvite urolithiasis is most notable in male dogs, with one study finding a decrease in submissions from 79% to 16%.15 The reduction in struvite urolithiasis in females over this time was less pronounced, from 97% of submissions to 68%.15 The reason for this decline is unknown, but the author speculates that calculolytic therapy with effective dissolution diets may reduce the numbers of struvite stones submitted for analysis.
Imaging
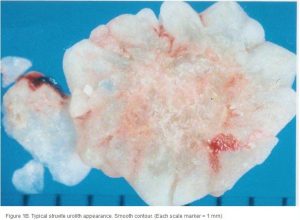
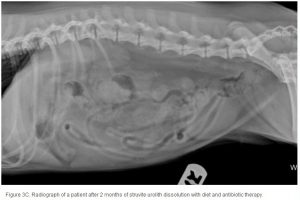
Conventional radiography has been shown to have a 2% false-negative rate in the diagnosis of struvite urolithiasis.22 The sensitivity is excellent for calculi that are >3 mm in diameter.23 Contrast studies using double-contrast cystography have been reported to improve sensitivity to 100%, even for calculi <1 mm.24 Abdominal ultrasonography provides equal sensitivity. Struvite mineral content can be predicted from survey radiographs with the following predictive values: urolith size >10 mm (90%), pyramid-shaped stones (100%), ovoid stones (80%), and smooth stones with blunt edges (75%; ; ). Struvite stones are radiopaque on survey radiographs but appear radiolucent with double-contrast studies using 80 mg/mL iodinated contrast medium administered via urinary catheter. 22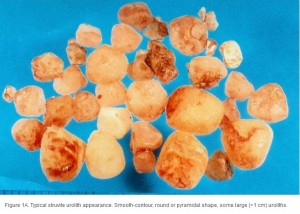
Treatment
While spontaneous dissolution has been reported, definitive therapy should be sought.23 Medical therapy includes elimination of urease-producing organisms, dietary modification, urease inhibition, and urinary acidification. FIGURE 2 summarizes the diagnosis and management of struvite urolithiasis.
 Dietary protein restriction decreases the amount ofurea available for urinary bacterial conversion to ammonium and, possibly, decreases urease production.25 Reducing urine pH increases the solubility of struvite crystals. A calculolytic diet (Hill’s s/d, Hill’s Pet Nutrition, Inc., Topeka, KS) is magnesium- and phosphorus-restricted, reduces urine pH and protein catabolism, and facilitates diuresis through sodium naturesis.3 Biochemical changes may be noticed with dietary adjustment, with a reduced serum urea nitrogen concentration being most reliably observed. Occasionally, mildly reduced albumin and phosphorus may be noted in the first month of therapy.25 Elevations in alkaline phosphatase activity are sometimes recognized, but the cause is unknown.23,25 A biochemical change compatible with dietary influences can aid in determination of owner compliance. While struvite crystalluria does not mean struvite calculi are present, this finding should resolve and can be used as a measure of owner compliance.23 Another diet (Royal Canin Veterinary Diet canine urinary SO 13, Royal Canin USA,
Dietary protein restriction decreases the amount ofurea available for urinary bacterial conversion to ammonium and, possibly, decreases urease production.25 Reducing urine pH increases the solubility of struvite crystals. A calculolytic diet (Hill’s s/d, Hill’s Pet Nutrition, Inc., Topeka, KS) is magnesium- and phosphorus-restricted, reduces urine pH and protein catabolism, and facilitates diuresis through sodium naturesis.3 Biochemical changes may be noticed with dietary adjustment, with a reduced serum urea nitrogen concentration being most reliably observed. Occasionally, mildly reduced albumin and phosphorus may be noted in the first month of therapy.25 Elevations in alkaline phosphatase activity are sometimes recognized, but the cause is unknown.23,25 A biochemical change compatible with dietary influences can aid in determination of owner compliance. While struvite crystalluria does not mean struvite calculi are present, this finding should resolve and can be used as a measure of owner compliance.23 Another diet (Royal Canin Veterinary Diet canine urinary SO 13, Royal Canin USA,
Inc., St. Charles, MO) has been shown to be effective in dissolving struvite calculi in an ex vivo model.26
The high sodium chloride concentration of calculolytic diets may be contraindicated for animals with hypertension and cardiac disease because it may cause volume expansion and exacerbation of these diseases. The reduced protein content may be inappropriate for young and old animals.3,23 The increased lipid content associated with protein restriction creates concerns regarding hypercholesterolemia, and concerns about the induction of pancreatitis in at-risk patients have also been expressed.3
Appropriate antibiotic therapy must be used in conjunction with calculolytic diets. Struvite stones that form in the presence of infection contain layers of bacterial colonies. Dissolution therapy results in a sustained release of viable bacterial pathogens as the outer mineral layers dissolve. It is important to perform urinalysis and culture every 4 weeks during therapy to evaluate for changing resistance patterns.25 Evidence of a persistently alkaline urine or active urine sediment may suggest ongoing infection. Successful stone dissolution with antibiotic therapy and a noncalculolytic diet has been described.26
Common causes of treatment failure include inability to control urinary tract infection, mixed or non-struvite stone composition, and poor dietary compliance. Calculus composition cannot be predicted reliably despite the radiographic characteristics described above or the associated urinary changes (alkaline urine, isolation of urease producing organism and struvite crystalluria). Non-struvite calculi and mixed calculi with >20% non-struvite composition are not dissolved by diet therapy.3
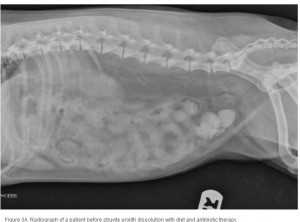
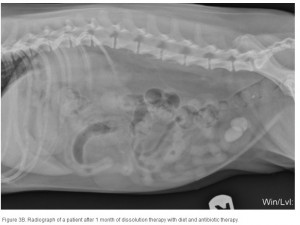
The average duration of dissolution therapy in dogs is 3 to 3.6 months, with a range from 2 to 5 months.27 The duration of therapy is individualized to the patient. Larger calculi have a reduced surface area relative to small calculi and therefore take longer to dissolve (; ; ). Clinical signs vary but often improve during the first 10 days of therapy in accordance with infection control.3 Clinical signs do not predict calculi dissolution, and serial objective monitoring (radiography or ultrasonography) is important to success. Serial radiographic assessments are recommended at 4-week intervals to detect changes in calculi number, location, and size.2,23 It is important that the same radiographic technique be used at each evaluation and that it is optimized for enhanced abdominal contrast. Medical therapy should be continued 1 month beyond radiographic clearance because calculi <3 mm cannot be accurately detected by radiography. Abdominal ultrasonography may provide a way of detecting small calculi without the invasiveness or risk of iatrogenic infections associated with contrast cystography.3 The authors recommend serial radiography until the calculi are no longer visible and focused ultrasonography before discontinuing dissolution therapy.
When dissolution dietary compliance cannot be achieved, using urinary acidification with D-L methionine could be considered. A recent abstract demonstrated efficacy at 100 mg/kg PO q12h when given with appropriate antibiotics even when diet was not changed.28
In 2000, medical dissolution of suspected struvite nephrolithiasis without dietary manipulation was described in two dogs receiving an intravenous amino acid infusion (Amiyu, Hoechst Marion Roussel Ltd, Tokyo, Japan).29
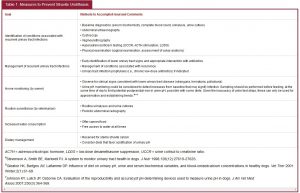
Clinical Pearl: Management of Recurrent Urinary Tract Infection
Evaluate for infection recurrence (relapsing and reinfection)
• Perform urine culture following discontinuation of antibiotics at 5 to 7 days and 1 month
Evaluate for causes of treatment failure
• Poor drug selection
• Poor drug absorption
• Poor owner compliance
• Bacterial sequestration (see internal risk factors)
Evaluate for cause of urine retention
Evaluate for external and nonstructural risk factors
• Diagnostics
o Vulvar conformational evaluation (e.g., recessed vulva)
o Perivulvar cytology (i.e., vulvovaginitis)
o Neurologic examination (i.e., urine retention)
Evaluate for internal risk factors
• Diagnostics
o Digital rectal and vaginal examination
o Abdominal ultrasound (e.g., neoplasia, etc.)
o Prosta
tic wash or third fraction collection (intact males)
o Cystoscopy (i.e., ectopic ureters)
o Contrast radiography
Evaluate for systemic risk factors
• Diagnostics
o Serum biochemistry
o Urinalysis
o Complete blood count
o Hyperadrenocorticism screening testing (when appropriate)
Eliminate complicating drugs (when possible)
• Immunosuppressive drugs
• Chemotherapy
Indication for urinary tract infection prophylaxis
• Evaluation for and elimination of all risk factors if there is persistent recurrence of infection
Mechanical Removal
Removal of small calculi can be performed with various nonsurgical techniques in select patient populations. These techniques include voiding urohydropropulsion, catheter-assisted retrieval, cystoscopic-assisted Ellik evacuator techniques, cystoscopic-assisted basket retrieval, lithotripsy, and percutaneous cystolithotomy.30–32
Minimally Invasive Techniques
Lithotripsy involves fragmenting stones mechanically using either extracorporeal shock waves or a laser. Cystoscopic retrieval and lithotripsy provide the clinician with minimally invasive options in the management of cystic, ureteral, and renal calculi, reducing patient morbidity. Large studies evaluating lithotripsy in the treatment of canine struvite nephrolithiasis or ureterolithiasis have not been performed. Only 33% of nephroliths and ureteroliths are composed of struvite in dogs.19
Laser lithotripsy techniques have been described for nephroliths, ureteroliths, and bladder and urethral calculi in dogs.32,33 Because direct contact with the calculus is necessary, a surgical or cystoscope-guided approach must be used. Stone composition does not influence the efficacy of fragmentation.33 Procedure times are shorter in female patients (median time: 42 min) compared with male patients (median time: 143 min) and for urethral calculi (median time: 70 min) compared with cystic calculi (median time: 105 min).32 Given the cost of lithotripsy equipment, limited accessibility, and procedure time, many clinicians prefer cystotomy or scope-assisted retrieval techniques. Minimally invasive techniques such as percutaneous cystolithomy have allowed visualized access to the bladder for retrieval of calculi and simultaneous access for mucosal biopsy/culture. Direct visualization of the bladder lumen can help reduce the likelihood of leaving calculi behind, which can occur with traditional cystotomy. The residual calculi rate with cystotomy has been reported to be as high as 20%.34
Surgical Therapy
Indications for surgical therapy include obstructive calculi of the urethra, renal pelvis, or ureters; failure of dissolution therapy; or unacceptable clinical signs associated with urolithiasis, or as a means of definitive treatment.35 Surgical intervention provides benefits such as reduced time to effective therapy, definitive diagnosis of stone type (via quantitative stone analysis), reduced risk of urinary obstruction, ability to collect samples for biopsy, and potential to recover renal function with resolution of obstructive calculi.
Procedures include urethrotomy, cystotomy, and laparoscopic-guided cystotomy for lower urinary tract calculi and ureterotomy, pyelolithotomy/nephrotomy, and nephrectomy for upper urinary tract calculi.31
Prevention
Increased water consumption is the mainstay of therapy in both people and animals to reduce supersaturation of urine with struvite constituents. However, recurrence of struvite calculi in dogs can be reduced by careful surveillance for bacterial urinary tract infections. Patients with recurrent urinary tract infections should be evaluated to identify and correct underlying risk factors, such as anatomic abnormalities (urachal diverticuli, vaginal recession, perivaginal dermatitis, neoplasia, polyps, strictures, granulomas). Testing for systemic diseases and risk factors associated with both overt and occult urinary tract infections should be performed. Predisposing factors for urinary tract infections include hyperadrenocorticism, diabetes mellitus, and immunosuppressive therapy.3,23,36–38 If no identifiable risk factors can be identified and corrected, idiopathic dysfunction of local urologic defense mechanisms is suspected.
An elevated urine pH may allow early suspicion of infection (). Infections should be treated aggressively before stones form. If recurrence of infection is noted and no identifiable risk factors can be identified, prophylaxis with urinary antiseptics or antibiotics can be considered. Urinary antiseptics are drugs that exert antimicrobial activity in the urine but little to no systemic effect; they include drugs like nitrofurantoin, methenamine, and nalidixic acid. Prophylactic antibiotic therapy includes the use of any other antibiotic class. Prophylactic antibiotics are typically given at one-half to one-third the standard dose once daily in the evening or, less commonly, “pulsed” for 1 week every month to prevent development of infection. Controlled studies of urinary antiseptics and/or prophylactic antibiotics in animals have not been performed.
Acetohydroxaminic acid (AHA), a competitive and noncompetitive inhibitor of the enzyme urease, can inhibit stone growth but is rarely used in dogs.39 Adverse effects are common, including hemolytic anemia, vomiting, anorexia, hyperbilirubinemia, and teratogenicity.40
Dietary intervention may be considered for sterile struvite calculi, as is commonly done in cats, although no clinical studies have been performed evaluating the efficacy of preventive diets in dogs. Dietary modification designed to achieve urinary pH <6.3 may be helpful because acidification may prevent struvite crystalluria, which could be important in preventing recurrent sterile struvite calculi, but this has not been critically evaluated. Some authors would consider urinary acidifiers (D,L-methionine) for prevention of struvite crystalluria when medical management fails to achieve optimal pH (<6.3).28
References
1. Dibartola SP, Chew DJ. Canine urolithiasis. Compendium Contin Educ Vet 1981;3(3):226-234.
2. Calabro S, Tudisco R, Bianchi S, et al. Management of struvite uroliths in dogs. Br Jour Nutr 2011;106:S191-S193.
3. McLean RJ, Downey J, Clapham L, Nickel JC. Influence of chondroitin sulfate, heparin sulfate, and citrate on Proteus mirabilis-induced struvite crystallization in vitro. J Urol 1990;144:1267-1271.
4. Osborne CA, Lulich JP, Polzin DJ, et al. Medical dissolution and prevention of canine struvite urolithiasis: twenty years of experience. Vet Clin North Am Small Anim Pract 1999;29(1):73-111.
5. Houston DM, Eaglesome H. Unusual case of foreign body-induced struvite urolithiasis in a dog. Can Vet J 1999;40:125-126.
6. Wang LP, Wong HY, Griffith DP. Treatment options in struvite stones. Urol Clin North Am 1997;24(1):149-162.
7. Adams LG, Syme HM. Canine lower urinary tract disease. In: Textbook of Veterinary Internal Medicine. 6th ed. Philadelphia, PA: WB Saunders; 1999:1850-1874.
8. Koehler LA, Osborne CA, Buettnera MT, et al. Canine uroliths: frequently asked questions and their answers. Vet Clin North Am Small Anim Pract 2008;39:161-181.
9. Albasan H, Lulich JP, Osborne CA, et al. Effects of storage time and temperature on pH, specific gravity, and crystal formation in urine samples from dogs and cats. J Am Vet Med Assoc 2003;222:176–179.
10. Bartges JW, Osborne CA, Pozin DJ. Recurrent sterile struvite urocystolithiasis in three related cocker spaniels. J Am Anim Hosp Assoc 1992;28:459-469.
11. Ling GV, Franti CE, Johnson BA, et al. Urolithiasis in dogs III: prevalence of urinary tract infection and interrelations of infection, age, sex, and mineral composition. Am J Vet Res 1998;59:643-649.
12. Houston DM, Moore AEP, Favrin MG, Hoff B. Canine urolithiasis: a look at over 16000 urolith submissions to the Canadian veterinary urolith centre from February 1998 to April 2003. Can Vet J 2004;45:225-230.
13. Sosnar M, Bulkova T, Ruzicka M. Epidemiology of canine urolithiasis in the Czech Republic from 1997 to 2002. J Small Anim Pract 2005;46;177-184.
14. Finco DR. Current status of canine urolithiasis J Am Vet Med Assoc 1971;158(3):327-335.
15. Ling GV, Franti CE, Johnson DL, et al. Urolithiasis in dogs I: prevalence of urinary tract infection and interrelations of infection, age, sex and mineral composition. Am J Vet Res 1998;59:624-629.
16. Ling GV, Franti CE, Johnson BA, et al. Urolithiasis in dogs II: breed prevalence, and interrelations of breed, sex, age, and mineral composition. Am J Vet Res 1998;9:630-642.
17. Ling GV, Thurmond MC, Choi YK, et al. Changes in proportion of canine urinary calculi composed of calcium oxalate or struvite in specimens analyzed from 1981 through 2001. J Vet Intern Med 2003;17:817-823.
18. Osborne CA, Lulich JP, Bartges JW, et al. Medical dissolution and prevention of canine and feline uroliths: Diagnostic and therapeutic caveats. Vet Rec 1990;127:369-373.
19. Ross SJ, Osborne CA, Lulich JP, et al. Canine and feline nephrolithiasis: epidemiology, detection and management Vet Clin North Am Small Anim Pract 1999;29:231-250.
20. Snyder DM, Steffey MA, Mehler SJ, et al. Diagnosis and surgical management of ureteral calculi in dogs: 16 cases (1990-2003). N Z Vet J 2004;52(1):19-25.
21. Jones BR, Omodo-Eluk AJ, Larkin H, et al. Canine uroliths: analysis of uroliths from dogs in Ireland. Irish Vet J 2001;54(12):629-632.
22. Feeney DA, Weichselbaum RC, Jessen CR, Osborne CA. Imaging canine urocystoliths: detection and prediction of mineral content. Vet Clin North Am Small Anim Pract 1999;29(1):59-72.
23. Seaman R, Bartges JW: Canine struvite urolithiasis. Compend Contin Ed Vet 2001;23(5):407-421.
24. Weichselbaum RC, Feeney DA, Jessen CR, et al. Urocystolith detection: comparison of survey, contrast radiographic and ultrasonographic techniques in an in vitro bladder phantom.Vet Radiol Ultrasound 1999;40(4):386-400.
25. Abdullahi SU, Osborne CA, Leininger FR, Fletcher TF. Evaluation of calculolytic diet in female dogs with induced struvite urolithiasis. Am J Vet Res 1984;45:1508-1519.
26. Rinkardt NE, Houston DM. Dissolution of infection-induced struvite bladder stones by using a noncalculolytic diet and antibiotic therapy. Can Vet J 2004;45:838-840.
27. Smith BH, Hynds W, Markwell PG. Ex vivo canine struvite stone dissolution. J Vet Intern Med 2001;15(3):301.
28. Bartges JW, Moyers T. Evaluation of D.L-methionine and antimicrobial agents for medical dissolution of spontaneously occurring infection-induced struvite urocystoliths in dogs. Proc 2010 ACVIM Forum.
29. Mishina M, Watanabe T, Fujii K, et al. Medical dissolution of struvite nephrolithiasis using amino acid preparation in dogs. J Vet Med Sci 2000;62(8):889-892.
30. Rawlings CA, Mahaffey MB, Barsanti JA, Canalis C. Use of laparoscopic-assisted cystoscopy for removal of urinary calculi in dogs. J Am Vet Med Assoc 2003;222(6):759-761.
31. McLoughlin MA, Bjorling DE. Ureters. In: Textbook of Small Animal Surgery. 3rd ed.Philadelphia, PA: WB Saunders; 2003:1619-1672.
32. Defarges A, Dunn M, Berent A. New alternatives for minimally invasive management of uroliths: lower urinary tract uroliths. Compend Contin Educ Vet 2013;35(1):E1-E7.
33. Wynn VM, Davidson EB, Higbee RG, et al. In vitro effects of pulsed holmium laser energy on canine uroliths and porcine cadaveric urethra. Lasers Surg Med 2003;33:243-246.
34. Grant DC, Harper TA, Were SR. Frequency of incomplete urolith removal, complications, and diagnostic imaging following cystotomy for removal of uroliths from the lower urinary tract in dogs: 128 cases (1994-2006)J Am Vet Med Assoc2010;236(7):763-766.
35. Domingo-Neumann RA, Ruby AL, Ling GV, et al. Ultrastructure of selected struvite-containing urinary calculi from dogs. Am J Vet Res 1996;57(9):1274-1287.
36. McGuire NC, Schulman R, Ridgway MD, Bollero G. Detection of occult urinary tract infections in dogs with diabetes mellitus. J Am Anim Hosp Assoc 2002;38(6):541-544.
37. Forrester SD, Troy GC, Dalton MN, et al. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J Vet Int Med 1999;13(6):557-560.
38. Torres SMF, Diaz SF, Nogueira SA, et al. Frequency of urinary tract infection among dogs with pruritic disorders receiving long-term glucocorticoid treatment. J Am Vet Med Assoc 2005;227(2):239-243.
39. Krawiec DR, Osborne CA, Leninger JR, Griffith DP. Effect of acetohydroxamic acid on dissolution of canine struvite uroliths. Am J Vet Res 1984;45:1266-1275.
40. Bailie NC, Osborne CA, Leininger JR, et al. Teratogenic effect of acetohydroxaminic acid in clinically normal beagles. Am J Vet Res 1986;47:2604-2611.